- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Amino Acids
Amino acids are the building blocks of proteins and play a central role in almost all biological processes. In the human body, they are essential for the formation of tissue, the repair of cells and the production of enzymes and hormones. There are 20 different amino acids, some of which are labelled "essential" because the body cannot produce them itself and they must therefore be obtained from food. Amino acids are also crucial for the metabolism, the immune system and the regulation of numerous physiological functions, such as the transport and storage of nutrients.
Amino acid analysis is therefore of great importance as it provides precise information on the quantity and profile of amino acids in biological samples, food or pharmaceutical products. These analyses are essential for assessing nutritional status, diagnosing metabolic disorders and ensuring the quality of food and food supplements. In medicine, amino acid analysis helps to detect imbalances that may indicate health problems, such as liver disease or genetic metabolic disorders. In the food industry, it contributes to the control and optimisation of protein content, which is particularly important for quality assurance and compliance with legal requirements.
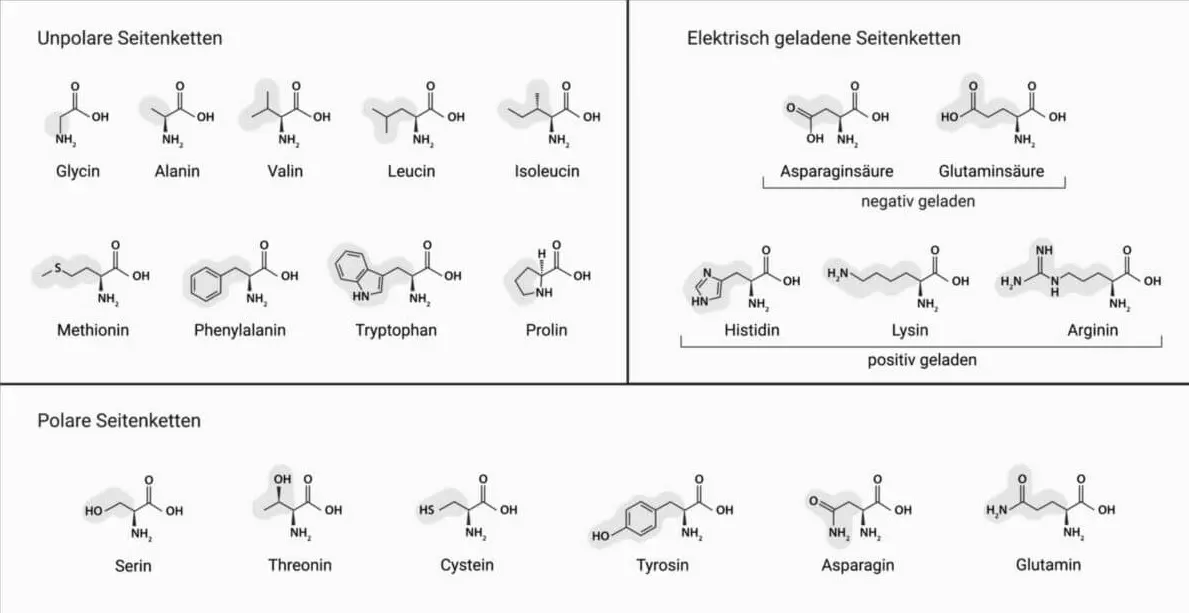
Overview of the different amino acids and the polarity of the side chains.
Products
Technical Data
Derivatisation of amino acids
In order to detect amino acids using fluorescence or to separate them using the reversed phase, amino acids must be derivatised. The classic method uses separation of the amino acids by ion exchange chromatography with subsequent post-column derivatisation to enable detection with a fluorescence detector. Ortho-phthaldialdehyde (OPA), 9-fluorenyl-methoxycarbonyl-chloride (FMOC-Cl), ninhydrin or fluorescamine can be used as reagents.
The most modern technique for the derivatisation of amino acids is pre-column derivatisation with ortho-phthaldialdehyde (OPA) and subsequent separation using reversed phase chromatography and subsequent fluorescence detection. The advantages here are the fast analysis times and the high sensitivities. However, a major disadvantage is that OPA only reacts with primary amines. This derivatisation method does not work for secondary amines such as proline.
The reagent 9-fluorenyl-methoxycarbonyl-chloride (FMOC-Cl) is therefore used for the pre-column derivatisation of secondary amines. After this derivatisation, the amino acids can also be separated using reversed-phase chromatography and detected using fluorescence.
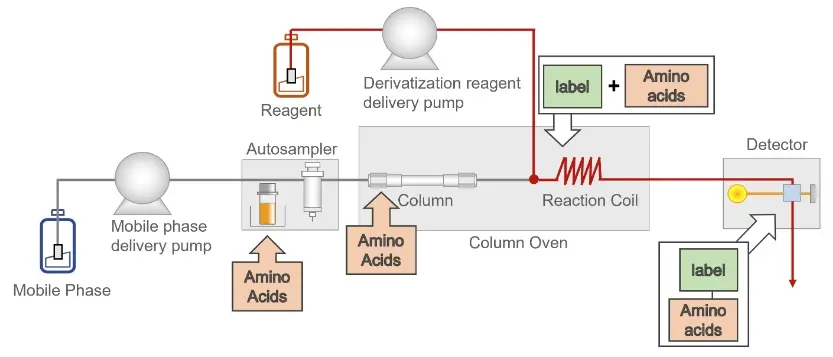
On-line and off-line derivatisation
Additional hardware is required for post-column derivatisation. The derivatisation reagent must be added to the amino acids in precise doses after the separation of the amino acids. This is achieved using an additional pump after the separation. After the reagent has been added, the amino acids need a certain amount of time to react. Therefore, a loop or a specially woven capillary is used to obtain a specific reaction time. An example setup can be found in the figure on the right.
An on-line solution can also be implemented for pre-column derivatisation. For this, only the arrangement of the post-column derivatisation has to be changed so that the derivatisation takes place before the column. However, this derivatisation can be carried out off-line, which means that there is no additional hardware requirement for the HPLC system. The sample is already derivatised before it is injected into the HPLC system.
Analysis of amino acids without derivatisation
It is also possible to separate and detect the amino acids without derivatisation. As in post-column derivatisation, the separation takes place using ion exchange or other special columns. The subsequent detection is then carried out using mass spectrometry (MS). The Intrada Amino Acid from Imtakt should be highlighted here as a special column for the separation of non-derivatised amino acids. With these columns it is possible to analyse the 20 amino acids within 10 minutes with MS/MS detection without derivatisation.
Applications
Underivatised amino acid analysis with Imtakt Intrada Amino Acid
Determination of 20 amino acids

Peak identities
Trp = tryptophan Phe = phenylalanine Tyr = tyrosine Met = methionine Leu = leucine , Ile= isoleucine Val = valine Glu = glutamic acid Pro = proline Thr = threonineAsp = aspartic acid Ala = alanine Ser = serine Gln = glutamine Lys = lysine Gly = glycine Asn = asparagine Cys = cysteine His = hystidine Arg = arginine
Test conditions
Column: Intarda Amino Acid 50 x 3 mm
Mobile phase A: acetonitrile /tetrahydrofuran /25mM ammonium formate /formic acid = 9 /75 /16 /0.3
Mobile phase B: Acetonitrile /100mM ammonium formate = 20 / 80
Gradient:
| Time (min) | %B |
| 0 | 0 |
| 2.5 | 17 |
| 6.5 | 100 |
Flow: 0.6 mL/min
Temperature: 35°C
Injection volume: 5 µL

Pre-column derivatisation of amino acids with OPA and FMOC using GL Sciences Inertsil ODS-4 HP
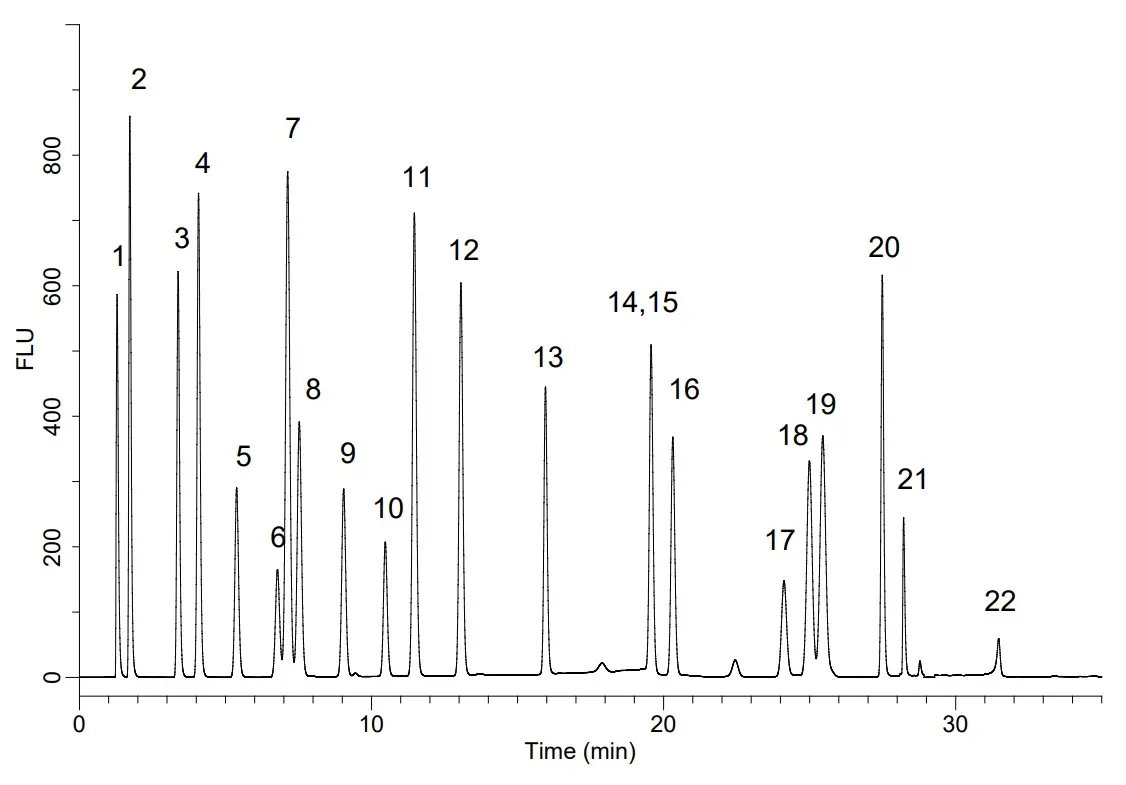
Peak identities
1. OPA-Aspartic Acid 2. OPA-Glutamic Acid 3. OPA-Asparagine 4. OPA-Serine 5. OPA-Glutamine 6. OPA-Histidine 7. OPA-Glycine 8. OPA-Threonine 9. OPA-Citrulline 10. OPA-Arginine 11. OPA-Alanine 12. OPA-GABA(4-aminobutanoic acid) 13. OPA-Tyrosine 14. OPA-Cys-Cys 15. OPA-Valine 16. OPA-Methionine 17. OPA-Tryptophan 18. OPA-Phenylalanine 19. OPA-Isoleucine 20. OPA-Leucine 21. OPA-Lysine 22. Fmoc-Proline
Test conditions
Column: Inertsil ODS-4 HP 150x3.0mm 3µm (5020-14005)
Mobile phase A: 45/40/15 acetonitrile/methanol/water
Mobile phase B: 20 mM K2HPO4 (pH 6.9)
Gradient:
| Time / min | %A | %B |
| 0 | 11 | 89 |
| 3 | 11 | 89 |
| 12 | 22 | 78 |
| 14 | 28 | 72 |
| 23 | 30 | 70 |
| 27 | 65 | 35 |
| 34 | 75 | 25 |
| 35 | 100 | 0 |
Flow rate: 0.7 mL/min
Temperature: 35 °C
Detection: Fluorescence excitation 350nm emission 450nm (0-29 min)
Excitation 266nm Emission 305nm (29-35 min)
Injection volume: 1 µL

Downloads
AMT
| Separation Mode | Product Line |
|---|---|
| HPLC - HILIC |
|
| HPLC - Reversed Phase |
|
ChromaNik
HPLC - Reversed Phase:
- SunShell RP-AQUA, 2.6µm
- SunShell C18, 2.6µm
- SunShell PFP, 2.6µm
- SunShell PFP&C18, 2.6µm
- SunShell Biphenyl, 2.6µm
- SunArmor NH2, 5µm
UHPLC - Reversed Phase:
- Sunniest C18-HT, 2µm
- ChromaNik SunArmor NH2 Branched-chain Amino Acids
- ChromaNik Sunniest C18-HT Amino Acids derivatized with OPA and FMOC
- ChromaNik Sunniest RP-AQUA Amino Acids LC/MS
- ChromaNik SunShell C18 Amino Acids derivatized with OPA and FMOC
- ChromaNik SunShell PFP, PFP&C18, RP-AQUA, Bipehynl Branched-chain Amino Acids
- ChromaNik SunShell RP-AQUA Amino Acids LC/MS
Concise
HPLC - IEX:
- AminoSep
- Amino Acid
GL Sciences
HPLC - Reversed Phase:
- Inertsil CN-3, 5µm
- Inertsil ODS-2, 5µm
- Inertsil ODS-4, 3µm
- Inertsil ODS-4 HP, 3µm
- Inertsil ODS-4V, 5µm
- InertSustain C18, 3µm
- InertSustain C30, 5µm
- InertSustain PFP HP, 3µm
- InertSustainSwift C18, 5µm
UHPLC - Reversed Phase:
- Inertsil ODS-4, 2µm
HPLC - HILIC:
- Inertsil Diol, 5µm
- InertSustain Amide, 5µm
GC:
- InertCap 5MS
- GL Sciences InertCap 5MS Analysis of Amino Acids as ter-Butyldimethylsilyl Derivatives
- GL Sciences Inertsil CN-3 Free Amino Acids and Organic Acids
- GL Sciences Inertsil Diol Analysis of Free Amino Acids
- GL Sciences Inertsil ODS-2 PTC-Amino Acids
- GL Sciences Inertsil ODS-2 PTH-Amino Acids
- GL Sciences Inertsil ODS-3V PTH-Amino Acids
- GL Sciences Inertsil ODS-4 Analysis of NBD-Amino Acids
- GL Sciences Inertsil ODS-4 Analysis of NBD-Amino Acids
- GL Sciences Inertsil ODS-4 Analysis of PTH-Amino Acids
- Gl Sciences Inertsil ODS-4 HP Analysis of Pre-column Derivatized Amino Acids
- GL Sciences Inertsil ODS-4 HP Analysis of PTH-Amino Acids
- GL Sciences Inertsil ODS-4V Analysis of 22 Amino Acids derivatized with NBD-F
- GL Sciences InertSustain Amide Analysis of Amino Acids
- GL Sciences InertSustain Amide Analysis of Amino Acids with HILIC
- GL Sciences InertSustain C18 Analysis of Metabolites - Amino Acids and their Related Compounds
- GL Sciences InertSustain C30 Analysis of 21 Amino Acids Derivatized with NBD-F
- GL Sciences InertSustain PFP HP Analysis of Amino Acids
- GL Sciences InertSustainSwift C18 Analysis of Amino Acids Derivatized with NBD-F
- GL Sciences InertSustainSwift C18 Analysis of Amino Acids Derivatized with NBD-F
- GL Sciences InertSustainSwift C18 Analysis of Amino Acids
Helix
HPLC - Mixed-Mode:
- Amaze HD, 5µm
- Amaze SC, 5µm
- Imtakt Intrada Amino Acid 55 Amino acids Standard Applications
- Imtakt Intrada Amino Acid Amino Acids in Soy Source Applications
- Imtakt Intrada Amino Acid Amino Acids Standard (Agilent) Applications
- Imtakt Intrada Amino Acid Amino Acids Standard (Shimadzu) Applications
- Imtakt Peptides, Proteins and Amino Acids Applications
- Imtakt PRTM Intrada Amino Acid Application
- Macherey-Nagel Lipodex E Identification of Amino Acidy by GC
- Macherey-Nagel Lipodex E Separation of Enantiomers of Amino Acid Methyl Esters
- Macherey-Nagel Nucleodur C18 ec Analysis of Amino Acids and Biogenic Polyamines by Pre-Column FMOC Derivatization
- Macherey-Nagel Nucleodur C18 ec Amino Acids OPA Derivatives
- Macherey-Nagel Nucleodur C18 ec Separation of Derivatized Amino Acids
- Macherey-Nagel Nucleosil C18 Amino Acid Analysis of Peptide Hydrolyzates by OPA Derivatives
- Macherey-Nagel Nucleosil C18 Enantiomeric Analysis of common Protein Amino Acids
- Macherey-Nagel Nucleosil Analysis of PTH-Amino Acids
- Nacalai Cosmocore 2.6C18 Analysis of DLDA-Amino Acids
- Nacalai Cosmocore 2.6C18 Analysis of DLDA Amino Acids by MS
- Nacalai Cosmocore 2.6C18 Analysis of DLDA-DL-Amino Acids
- Nacalai Cosmosil 3C18 AR-II Analysis of DVDA-DL-Amino Acids
- Nacalai Cosmosil 3C18-EB Analysis of Butyric Acid Derivatives
- Nacalai Cosmosil 3PBr Analysis of DLDA Amino Acids
- Nacalai Cosmosil 5C18 AR-II Analysis of DLDA-DL-Amino Acids
- Nacalai Cosmosil Cholester Analysis of DLDA-DL-Amino Acids
- Osaka-Soda Capcell Core AQ S2.7 NBD-Amino Acids
- Osaka-Soda Capcell Core C18 S2.7 NBD Amino Acids
- Osaka-Soda Capcell PAK ADME-HR S2 Amino Acids
- Osaka-Soda Capcell PAK C18 MG S5 NBD Amino Acids
- Osaka-Soda Capcell PAk C18 MG S5 NBD-Amino Acids
- Osaka-Soda Capcell PAK C18 UG120 S5 Acetyl Amino Acids
- Osaka-Soda Capcell PAK UG120 S5 Analysis of 19 Dabsyl-Amino Acids
- Osaka-Soda Capcell PAK C18 UG120 S5 PTH-Amino Acids
- Osaka-Soda Capcell PAk C18 UG80 S5 PTH-Amino Acids
Sepax
HPLC - Reversed Phase:
- GP-C18, 5µm
- Shimadzu Shim-pack Amino-Li Analysis of Free Amino Acids under Li Type Condition
- Shimadzu Shim-pack Amino-Li Analysis of Amino Acids in Fermented Food and Drinks
- Shimadzu Shim-pack Amino-Na Analysis of Proteinogenic Amino Acids under Na Type Conditions
- Shimadzu Shim-pack Amino-Na Analysis of Food using Nexera Post-Column Amino Acid Analysis System
- Shimadzu Shim-pack UF-Amino Ultra Fast Amino Acid Analysis System
- Shimadzu Shim-pack XR-ODS Ultra Fast Amino Acid Analysis System
- Shimadzu Shim-pack XR-ODSII High-speed Analysis of Amino Acids
- Shimadzu Shim-pack XR-ODSII Analysis of Amino Acids Using Automated Pretreatment Function
- Shimadzu Shim-pack XR-ODSII Analysis of Amino Acids by Pre-column Derivatization Methods
- Shimadzu Shim-pack XR-ODSII Selection of Detector in Analysis of Amino Acids using Pre-column Derivatization
- Shimadzu Shim-pack XR-ODSII Analysis of Amino Acid by Pre-column Derivatization
- Shimadzu Shim-pack XR-ODSII Analysis of Amino Acids by Automated Pretreatment
Shodex
HPLC - Reversed Phase:
- RSpak NN-814
HPLC - HILIC:
- HILICpak VC-50 2D
- Asahipak NH2P-40 2D
HPLC - Ion chromatography:
- IC YS-50
- CXpak P-421S
Thermo
HPLC - Reversed Phase:
- Acclaim PolarAdvantage, 5µm
UHPLC - Reversed Phase:
- Acclaim PA2, 2.2µm
HPLC - HILIC:
- Accucore-150-Amide-HILIC, 2.6µm
HPLC - IEX:
- AminoPac PA10
GC:
- TRACE TR-5
- Thermo Acclaim PA2 Analysis of Pierce Amino Acid Standard H
- Thermo Acclaim PolarAdvantage Amino Acids by Automated OPA Derivatization
- Thermo Accucore-150-Amide-HILIC Underivatized Amino Acid Analysis in Wine
- Thermo AminoPac PA10 Gradient Method for the AAA-Direct Separation of Amino Acids and Carbohydrates
- Thermo AminoPac PA10 Determination of Amino Acids in Cell Cultures and Fermented Broths
- Thermo TRACE TR-5 GC Analysis of Derivatized Amino Acids
Tosoh
HPLC - Reversed Phase:
- TSKgel ODS-100V, 5µm
- Waters AccQ Tag Ultra Amino Acid Analysis unsing Andrew+ Automated Preparation
- Waters AccQ Tag Ultra Determination of Amino Acids in Beers
- Waters AccQ Tag Ultra Monitoring Cell Culture Media for Amino Acids
- Waters AccQ Tag Ultra High-Throughput Amino Acid Analysis Using Hamilton Automated Preparation
- Waters AccQ Tag Ultra High-Throughput Amino Acid Analysis using Tecan Automated Preparation
- Waters AccQ Tag Ultra Rapid Analysis of Amino Acids in Wine
- Waters AccQ Tag Ultra C18 Instrument Considerations for Reliable Amino Acid Analysis
- Waters Acquity UPLC HSS T3 A Validated Method for the Quantification of Amino Acids in Mammalian Urine
- Waters Acquity Premier HSS T3 Quantification of Underivatized Amino Acids in Cell Culture Media
- Waters Amino Acid Analysis Application Notebook
- Waters Cortecs UPLC C18 Analysis of 20 Amino Acids using the Kairos Amino Acid Kit
- Waters Cortecs UPLC C18 Analysis of 45 Amino Acids using the Kairos Amino Acid Kit
- Waters MassTrak AAA Analysis of Physiological Amino Acids
- Waters XBridge Premier BEH Amide HILIC-MS Separation of 17 Free Amino Acids
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.
You will find:
Write us a message and we will get back to you as soon as possible.