Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Method Development
Method development in chromatography is a systematic process to create an analytical method that separates sample components specifically, reliably and efficiently. This includes selecting the appropriate type of chromatography, determining the optimum column and mobile phase conditions and setting the detector parameters. The aim is to develop a method that offers a high resolution of the analytes, delivers reproducible results and fulfils the analytical requirements. This process requires in-depth knowledge of the chemical properties of the analytes and the interactions with the stationary phase.
MZ-Analysentechnik GmbH gives you access to almost the entire range of stationary phases available worldwide so that you always have the right column for your analysis. We will be happy to help you select the right column for your needs.
The steps of a typical method development could be as follows:
- Selection of the separation technique (RP, NP, HILIC, SEC, IEX,...)
- Selection of the column dimension
- Selection of the stationary phase
- Selection of the mobile phase
- Adjustment of the pH value of the mobile phase with a suitable buffer reagent (if required)
- Carrying out some isocratic or gradient runs to determine basic parameters
- Further optimisation of the parameters, e.g. by varying
- the temperature
- the buffer or ion pair reagent
- the flow rate
- the gradient
Some manufacturers provide helpful literature on the subject of method development, which makes it easier for the user to find the optimum conditions for his separation problem. Please take a look at the brochures below. If you have any questions on the subject of method development or column selection, please do not hesitate to contact us.
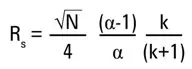
Technical information
The aim of chromatographic method development is to separate a certain number of analytes in the shortest possible time and with the best possible resolution. At the same time, the methods should be reproducible and robust so that identical results can be obtained at different times and in different places.
The resolution (Rs) of a separation is described by the following equation:
The resolution is therefore influenced by the efficiency N, the selectivity α and the retention k.
The efficiency N of a column depends on its length, particle size and particle shape, among other things. It is described by the theoretical plate number of a column. The selectivity α is mainly influenced by the type of stationary phase and the mobile phase. The retention k can also be influenced by the type of stationary phase and by the elution power of the mobile phase.
For example, doubling the number of plates (either by doubling the column length or reducing the particle diameter) increases the resolution by a factor of root 2, i.e. by only about 40%. However, this increases the analysis time and/or the back pressure considerably. It should also be borne in mind that halving the particle diameter quadruples the back pressure.
The graph of resolution versus efficiency, selectivity and retention shows that the resolution of a separation is most strongly influenced by selectivity.
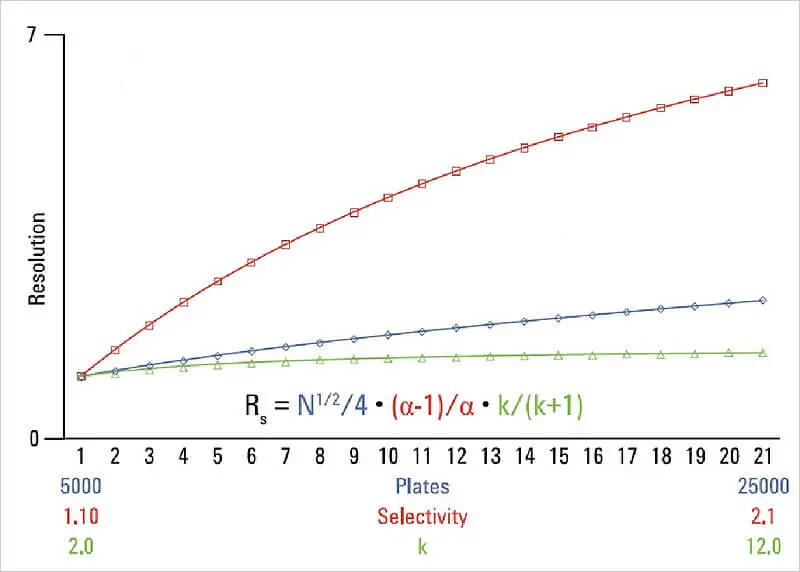
For this reason, the selectivity of the stationary phase is one of the most useful parameters for setting the HPLC separation selectivity and thus optimising the resolution. The methods are easier to reproduce, especially between laboratories, if the selectivity is derived from the stationary phase when using simple mobile phases. If the stationary phase has the right selectivity for the given separation problem, a lot of work can be saved during the subsequent optimisation.
Why is it important to check the pH value?
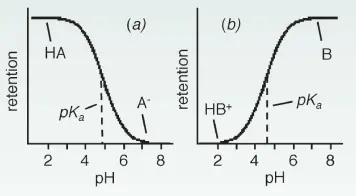
Ionisation of the Analytes
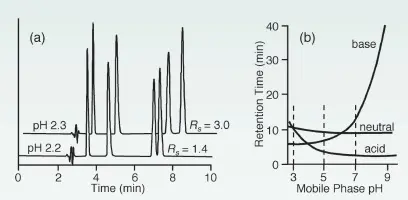
Ionisable compounds (such as acids or bases) are either ionised or neutral at different pH values. If the pH value differs from the pka value of a compound by more than 2 units, then it is 99% ionised or neutral. If this compound is neutral, then it is also more hydrophobic, which leads to a longer retention time in reversed-phase chromatography. This is the case for acids at pH < pka and for bases at pH > pka. If the pH value is close to the pka value of a component, even small changes in the pH value can lead to large differences in the degree of ionisation and therefore also retention.
Ionisation of the Stationary Phase
In addition to the analytes, the pH value also influences the column. Firstly, the pH value must lie within the limits of the column. For silica gels this is usually 2<pH<8. Another important aspect is the deprotonation of the free silanol groups. In older "type A" silica gels, the pka value of these free silanols is 4-5, which means that the ionization of the stationary phase can change significantly in this pH range. If the free silanols are deprotonated, the negatively charged groups lead to cation exchange and strong tailing for bases. For this reason, newer "type B" silica gels are usually used nowadays, which have a pKa value of >7 and generally also show significantly more symmetrical peaks for bases.
Start of Method Development
Conclusion: Due to the ionisation of analytes and the stationary phase, it is recommended to start method development at a pH value of 2-3. At this pH value, most organic acids are neutral and the bases are charged. At the same time, the stationary phase is also neutral and retards the acids more than the bases. As a result, bases elute first. If the pka values of the analytes are known, it also makes sense to use a pH value two units away from the pka values in order to obtain a robust method.
Which buffers can be used?
A buffer is most effective in the range of ±1 pH around its pka value. However, as only a small amount of sample is injected in chromatography, a pH range of ±2 around the pka value may be sufficient.
| Buffer | pka | pH range | LC-MS compatible |
| Phosphate (pk1) | 2.15 | 1.1 - 3.1 | - |
| Phosphate (pk2) | 7.20 | 6.2 - 8.2 | - |
| Phosphate (pk3) | 12.3 | 11.3 - 13.3 | - |
| Acetate | 4.8 | 3.8 - 5.8 | + (as ammonium acetate) |
| Trifluoroacetic acid (TFA) | 0.3 | 2.0 | + (but suppresses ionisation) |
| Phosphoric acid | 2.15 | 2.0 | - |
| Formic acid | 3.75 | 2.7 | + |
| Ammonium formate | 3.7 | 2.7 - 4.7 | + |
| Ammonium bicarbonate | 7.6 | 6.6 - 8.6 | + |
| Borate | 9.3 | 8.3 - 10.3 | - |
 Avantor ACE HPLC Buffer Solution Guide
Avantor ACE HPLC Buffer Solution Guide