- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Normal Phase Chromatography
Normal Phase Chromatography is the original type of liquid chromatography, as it was also used in the early years of chromatography. It can sometimes also be referred to as adsorption chromatography if the separation mechanism is based on adsorption due to the stationary phase selected. Normal phase chromatography is generally characterised by stationary phases with polar surface properties and a mobile phase with a non-polar character.
Products
Technical Data
Basics
The stationary phases of normal phase chromatography
Unmodified silica gels or aluminium oxide particles are often used as stationary phases, as the adsorption effect is the main separation mechanism with these materials and can therefore be referred to as "true" adsorption chromatography.
Other materials that can be used in normal phase mode (i.e. with non-polar mobile phases) are specially modified silica gels (cyano, amino, diol, nitro), which will not be discussed in detail here. More detailed information can be found under the heading "Medium Polar Phase Chromatography".
Mobile phases of normal phase chromatography
Almost all common organic solvents and their mixtures can be used as mobile phases in normal phase chromatography, as long as they are not strong acids or bases. Aliphatic hydrocarbons, such as hexane or heptane, have the weakest elution power. As the polarity of the solvent increases, so does its elution power. Polar solvents used include acetic acid ethyl ester, acetone, THF or various aliphatic alcohols.
What is the separation in normal phase chromatography based on?
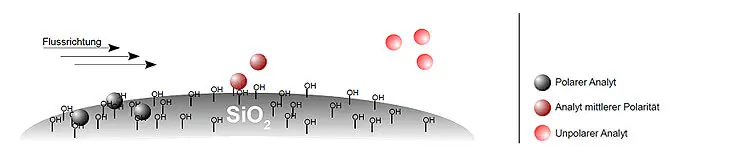
Due to the ability of the stationary phase to form hydrogen bonds, electrostatic and other intermolecular interactions with the analytes, retention in normal phase chromatography is based on the different degrees of adsorption of the analytes on the stationary phase. Polar analytes are therefore retained more strongly than non-polar analytes, as they are more able to enter into polar interactions with the stationary phase.
Other manufacturers of normal phase chromatography columns:
-

Agela Technologies
-

Agilent Technologies
-
![[English:] [English:]](https://cms.mz-at.de/fileadmin/_processed_/f/d/csm_logo_avantor-ace_web23_w600-h225px_7d5acb3b41.webp)
Avantor-ACE
-

Bischoff
-

Dikma
-

Dr. Maisch
-

GL Sciences
-

Hamilton
-

Imtakt
-

Macherey-Nagel
-

Merck
-

Merck Supelco (Sigma-Aldrich)
-

MicroSolv
-

Nacalai Tesque
-

Nouryon - Kromasil
-

Osaka Soda (Shiseido)
-

Perkin-Elmer
-

Restek
-

Sepax
-

Shodex (Resonac)
-

SIELC
-

SMT-Separation Methods Technologies
-

Sumika Chemicals
-

Thermo Scientific
-

Trajan Scientific (SGE)
-

Waters
-

Welch Materials
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.