- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Ion Exclusion Chromatography
Ion exclusion chromatography is a specialised technique of Ion exchange chromatography which is often used for analysing sugars, organic acids and alcohols.
Products
Technical Data
Basics
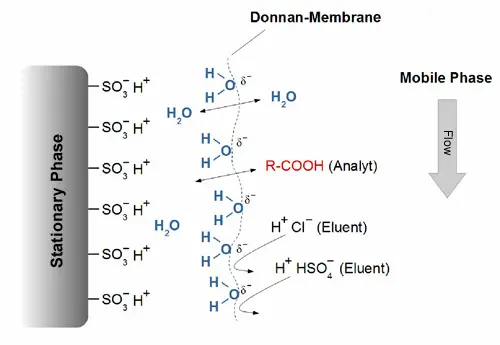
A completely sulphonated cation exchanger is often used as the stationary phase. A hydrate shell is formed around the sulphonic acid groups using low-concentration, strong aqueous acids as the mobile phase. This is bounded by an (imaginary) partially negatively charged membrane, which is also referred to in the literature as a Donnan membrane. The stationary phase is therefore only accessible to uncharged, non-dissociated molecules such as water. Analytes with a strongly acidic character are excluded, as these are still partially deprotonated under the conditions of the mobile phase and are therefore repelled by the Donnan membrane.
Excluded ions cannot penetrate the pore system of the stationary phase and are the first to be eluted. Weak acids, on the other hand, are protonated in the solvent, i.e. they are uncharged. They can therefore pass through the Donnan membrane and go to the surface of the stationary phase, where they are separated. Ion-exclusion chromatography is often used for the simultaneous separation of sugars, organic acids and alcohols, e.g. in fermentation analysis.
Manufacturers and columns of ion exclusion chromatography
Concise Separations offers a very wide range of high quality columns for ion exclusion chromatography. Materials with different degrees of cross-linking and particle sizes are available, covering a wide range of applications.
Shodex ion exclusion columns are proven columns for analysing organic acids, alcohols and carbohydrates.
Sepax Carbomix H is a column for ion exclusion chromatography.
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.