- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Ion Exchange Chromatography
Ion exchange chromatography (IEX) is used to separate and analyse charged or ionisable molecules. A distinction is made between anion and cation exchangers, and also between strong and weak exchangers.
- SAX: Strong Anion Exchanger
- WAX: Weak Anion Exchanger
- SCX: Strong Cation Exchanger
WCX: Weak Cation Exchanger
Ion exchange chromatography is often used as a bioseparation technique for proteins and antibodies. Proteins are separated according to their net or surface charge, which is dependent on the pH value and the ionic strength of the mobile phase. Other areas of application include oligonucleotides, sugars, RNA and DNA molecules and more.
Ion chromatography and ion exclusion chromatography are two special cases of ion exchange chromatography with their own column technologies for specific applications.
Products
Technical Data
Basics
An elution sequence for ion exchange chromatography (IEX) is often difficult to predict because the elution or retention of molecules depends on many parameters. Important parameters include the type of ion exchanger used, the pH value, the ionic strength and the type of counterion in the mobile phase, as well as the electronic properties of the analytes.
The surface of the stationary phases carry ionic groups, which are neutralised to the outside by mobile counterions of the mobile phase.

The analyte molecules compete with each other and with the counterions present in the mobile phase for a place on the surface of the stationary phase. As a rule, more strongly charged analyte molecules are also more strongly retarded than those that only have weak charges or are neutral.

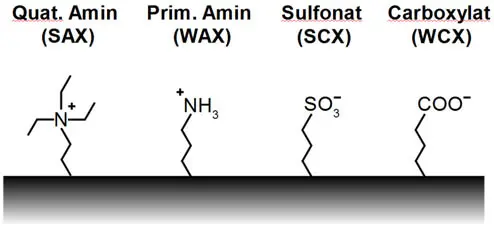
Anion exchangers carry positive charges on the surface, e.g. quaternary or primary ammonium groups, in order to bind anions. Cation exchangers therefore carry negative charges on the surface,
e.g. sulphonates or carboxylates, in order to bind cations (see figure for examples).
Ion exchange chromatography manufacturers and columns
1. Columns for cation exchange chromatography
| Manufacturer | Name | Base material | Modification | Pore size | Particle size | pH range |
| Tosoh Bioscience | TSKgel CM-2SW | Silica gel | Carboxymethyl (Na+) | 125 Å | 5 µm | 2.0-7.5 |
TSKgel CM-3SW | Silica gel | Carboxymethyl (Na+) | 250 Å | 10 µm | 2.0-7.5 | |
TSKgel SP-2SW | Silica gel | Sulfopropyl (Na+) | 125 Å | 5 µm | 2.0-7.5 | |
TSKgel CM-5PW | Polymethacrylates | Carboxymethyl (Na+) | 1000 Å | 10 & 13 µm | 2.0-12.0 | |
TSKgel SP-5PW | Polymethacrylates | Sulfopropyl (Na+) | 1000 Å | 10, 13 & 20 µm | 2.0-12.0 | |
TSKgel SP-NPR | Polymethacrylates | Sulfopropyl (Na+) | non-porous | 2.5 µm | 2.0-12.0 | |
TSKgel SP-STAT | Polymethacrylates | Sulfopropyl (Na+) | non-porous | 7 & 10 µm | 3.0-10.0 | |
TSKgel CM-STAT | Polymethacrylates | Carboxymethyl (Na+) | non-porous | 7 & 10 µm | 3.0-10.0 | |
TSKgel Bioassist S PEEK | Polymethacrylates | Sulfopropyl (Na+) | ~1300 Å | 7 & 13 µm | 2.0-12.0 | |
TSKgel SCX (Na+) | PS/DVB | Sulphonic acid (Na+) | 60 Å | 5 µm | 1.0-14.0 | |
TSKgel SCX (H+) | PS/DVB | Sulphonic acid (H+) | 60 Å | 5 µm | 1.0-14.0 | |
| Shodex | IEC SP-825 | Polymethacrylates | Sulfopropyl | 5000 Å | 8 µm | 2.0-12.0 |
IEC SP-2025 | Polymethacrylates | Sulfopropyl | 5000 Å | 20 µm | 2.0-12.0 | |
IEC SP-FT 4A | Polymethacrylates | Sulfopropyl | non-porous | 2.7 µm | 2.0-12.0 | |
IEC CM-825 | Polymethacrylates | Carboxymethyl | 5000 Å | 8 µm | 2.0-12.0 | |
IEC CM-2025 | Polymethacrylates | Carboxymethyl | 5000 Å | 20 µm | 2.0-12.0 | |
Asahipak ES-502C 7C | Polymethacrylates | Carboxymethyl | 2000 Å | 9 µm | 2.0-12.0 | |
Asahipak ES-502C 20C | Polymethacrylates | Carboxymethyl | 2000 Å | 13 µm | 2.0-12.0 | |
CXpak P-421S | PS/DVB | Sulphonic acid (Na+) | N/A | 6 µm | 3.0-14.0 | |
CXpak P-G | PS/DVB | Sulphonic acid (Na+) | N/A | 6 µm | 3.0-14.0 | |
| Sepax | Proteomix SCX | PS/DVB with neutral | Sulphonate | non-porous | 1.7, 3, 5 & 10 µm | 2.0-12.0 |
Proteomix WCX | PS/DVB with neutral | Carboxylates | non-porous | 1.7, 3, 5 & 10 µm | 2.0-12.0 | |
Antibodix WCX | PS/DVB with neutral | Carboxylates | non-porous | 1.7, 3, 5 & 10 µm | 2.0-12.0 | |
| GL Sciences | InertSil CX | Silica Gel | Benzenesulfonic acid | 100 Å | 5 µm | 2.0-7.5 |
| Macherey nail | Nucleogel SCX | Polymethacrylates | Sulphonates | 1000 Å | 8 µm | 1.0-13.0 |
| Separation Methods Technologies | SMT SCX | Silica Gel | Sulfonic acid | 100 Å, 300 Å | 5 & 10 µm | 2.0-7.5 |
SMT WCX | Silica gel | Carboxylic acid | 100 Å, 300 Å | 5 & 10 µm | 2.0-7.5 | |
| Supelco | Discovery Bio PolyMA-SCX | Polymethacrylates | Sulfopropyl (Na+) | 1000 Å | 5 µm | 1.0-13.0 |
| Thermo Scientific | BioBasic SCX | Silica Gel | Sulfonic acid | 300 Å | 5 µm | 2.0-7.5 |
ProPac SCX-10 | Ethylvinylbenzene crosslinked | Sulfonic acid | non-porous | 10 µm | 2.0-12.0 | |
ProPac SCX-20 | Ethylvinylbenzene crosslinked | Sulfonic acid | non-porous | 10 µm | 2.0-12.0 | |
ProPac WCX-10 | Ethylvinylbenzene crosslinked | Carboxylic acid | non-porous | 10 µm | 2.0-12.0 | |
ProPac Elite WCX | DVB particles with | Carboxylic acid | non-porous | 5 µm | 2.0-12.0 | |
ProSwift SCX-1S | Polymethacrylate | Sulphonic acid | N/A | monolithic | 2.0-12.0 | |
ProSwift WCX-1S | Polymethacrylate | Carboxylic acid | N/A | monolithic | 2.0-12.0 | |
MAbPac SCX-10 | Polydivinylbenzene | Sulfonic acid | non-porous | 3, 5, & 10 µm | 2.0-12.0 |
2. Columns for anion exchange chromatography
| Manufacturer | Name | Base material | Modification | Pore size | Particle size | pH range |
| Tosoh Bioscience | TSKgel BioAssist Q | Polymethacrylates | Polyamines (Cl-) | ~4000 Å | 10 & 13 µm | 2.0-12.0 |
TSKgel SuperQ-5PW | Polymethacrylates | Trimethyl-amino (Cl-) | 1000 Å | 10 & 13 µm | 2.0-12.0 | |
TSKgel DEAE-5PW | Polymethacrylates | Diethylaminoethyl (Cl-) | 1000 Å | 10, 13 & 20 µm | 2.0-12.0 | |
TSKgel Q-STAT | Polymethacrylates | Trimethyl-amino (Cl-) | non-porous | 7 & 10 µm | 3.0-10.0 | |
TSKgel DNA-STAT | Polymethacrylates | Trimethyl-amino (Cl-) | non-porous | 5 µm | 3.0-10.0 | |
TSKgel DEAE-NPR | Polymethacrylates | Diethylaminoethyl (Cl-) | non-porous | 2.5 µm | 2.0-12.0 | |
TSKgel DNA-NPR | Polymethacrylate | Proprietary (ClO4-) | non-porous | 2.5 µm | 2.0-12.0 | |
TSKgel DEAE-2SW | Silica gel | Diethylaminoethyl (H2PO4-) | 125 Å | 5 µm | 2.0-7.5 | |
TSKgel DEAE-3SW | Silica gel | Diethylaminoethyl (Cl-) | 250 Å | 10 µm | 2.0-7.5 | |
TSKgel SUGAR AXI | PS/DVB | Trimethyl-amino (HBO3-) | 60 Å | 8 µm | 1.0-14.0 | |
TSKgel SUGAR AXG | PS/DVB | Trimethyl-amino (HBO3-) | 60 Å | 10 µm | 1.0-14.0 | |
TSKgel SAX | PS/DVB | Trimethyl-amino (Cl-) | 60 Å | 5 µm | 1.0-14.0 | |
| Shodex | IEC QA-825 | Polymethacrylate | Quat. Ammonium | 5000 Å | 12 µm | 2.0-12.0 |
IEC QA-2025 | Polymethacrylates | Quat. Ammonium | 5000 Å | 20 µm | 2.0-12.0 | |
IEC DEAE-825 | Polymethacrylates | Diethylaminoethyl | 5000 Å | 8 µm | 2.0-12.0 | |
IEC DEAE-2025 | Polymethacrylates | Diethylaminoethyl | 5000 Å | 20 µm | 2.0-12.0 | |
AXpak WA-624 | Polymethacrylates | Diethylaminoethyl | 2000 Å | 10 µm | 3.0-12.0 | |
Asahipak ES-502N 7C | Polyvinyl alcohol | Diethylaminoethyl | 2000 Å | 9 µm | 2.0-12.0 | |
Asahipak ES-502N 20C | Polyvinyl alcohol | Diethylaminoethyl | 2000 Å | 13 µm | 2.0-12.0 | |
| Sepax | Proteomix SAX | PS/DVB with neutral | Quat. Ammonium | non-porous | 1.7, 3, 5 & 10 µm | 2.0-12.0 |
Proteomix WAX | PS/DVB with neutral | Diethylaminoethyl | non-porous | 1.7, 3, 5 & 10 µm | 2.0-12.0 | |
Glycomix SAX | Hydrophilic polymer | Quat. Ammonium | N/A | N/A | 2.0-12.0 | |
| GL Sciences | InertSil AX | Silica Gel | Diethylaminopropyl | 100 Å | 5 µm | 2.0-7.5 |
| Macherey nail | Nucleogen DEAE | Silica gel | Diethylaminoethyl | 60, 500, 4000 Å | 7 µm | 2.0-7.5 |
Nucleogel SAX | Quaternised PEI | Quat. Ammonium | 1000 Å | 8 µm | 1.0-13.0 | |
| Separation Methods Technologies | SMT SAX | Silica Gel | Quat. Ammonium | 100, 300 Å | 5 & 10 µm | 2.0-7.5 |
SMT WAX | Silica gel | Polyethyleneimine | 100, 300 Å | 5 & 10 µm | 2.0-7.5 | |
| Supelco | Discovery Bio PolyMA-WAX | Polymethacrylates | Diethylaminoethyl | 1000 Å | 5 µm | 2.0-11.0 |
| Thermo Scientific | Hypersil Gold AX | Silica Gel | Polyethyleneimine | 175 Å | 1.9, 3 & 5 µm | 2.0-8.0 |
HyperSil Gold SAX | Silica Gel | Quat. Ammonium | 175 Å | 1.9, 3 & 5 µm | 2.0-8.0 | |
BioBasic AX | Silica Gel | Polyethyleneimine | 300 Å | 5 µm | 2.0-8.0 | |
ProPac SAX-10 | Ethylvinylbenzene crosslinked | Quat. Ammonium | non-porous | 10 µm | 2.0-12.0 | |
ProPac WAX-10 | Ethylvinylbenzene crosslinked | Tertiary amines | non-porous | 10 µm | 2.0-12.0 | |
ProSwift SAX-1S | Polymethacrylate | Quat. Ammonium | N/A | monolithic | 2.0-12.0 | |
ProSwift WAX-1S | polymethacrylates | Diethylaminoethyl | N/A | monolithic | 2.0-12.0 | |
DNAPac PA100 | Pellicular polymer beads | Quat. Ammonium | non-porous | 13 µm | 2.0-12.5 | |
DNAPac PA200 | Pellicular polymer beads | Quat. Ammonium | non-porous | 8 µm | 2.0-12.5 | |
DNAPac PA200RS | Pellicular polymer beads | Quat. Ammonium | non-porous | 4 µm | 2.0-12.5 | |
DNASwift SAX-1S | Polymethacrylate | Quat. Ammonium | N/A | monolithic | 6.0-12.4 |
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.