- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Chiral Chromatography
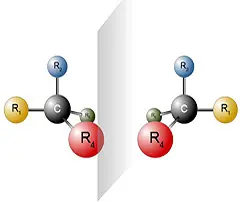
Chiral chromatography is a method for separating mixtures of enantiomers. Enantiomers are molecules in which the image and mirror image do not coincide. Enantiomers differ in their properties only in the direction of rotation of linearly polarised light. In all other chemical and physical properties they show no difference. For this reason, enantiomers cannot be separated using "conventional" HPLC techniques.
The main area of application is in pharmaceutical research and industry in order to obtain biologically active substances and drugs enantiomerically pure. This is extremely important as enantiomers can have completely different pharmacological effects. It is therefore necessary for drug development that the target compounds are enantiomerically pure and are tested separately for their efficacy and toxicity.
Products
Technical Data
Fundamentals of chiral chromatography
What possibilities are there for enantiomers via HPLC?
- Chirality is "added" to the mobile phase - e.g. by adding a chiral reagent. This creates diastereomeric complexes between the sample molecules and the molecules of the chiral reagent, which migrate through the separation column at different speeds. The stationary phase remains unchanged, i.e. it is achiral.
- The enantiomers are reversibly derivatised, i.e. chemically reacted, with a suitable chiral compound. This produces diastereomers that can be separated on conventional HPLC phases. After separation, the derivatives are cleaved again, whereby care must be taken to ensure that no racemisation occurs under the reaction conditions.
- A specially modified, chiral phase is used as the stationary phase. The mobile phase remains unchanged. The enantiomers to be separated interact with the chiral stationary phase to varying degrees and thus leave the separation column at different times.
The last of the options mentioned (number 3) is generally referred to as "chiral HPLC". It is the easiest of the three variants to carry out and the probability of a successful separation is almost guaranteed due to the large number of stationary phases available.
Which stationary phases are used for chiral HPLC?
The stationary phases for chiral HPLC (chiral stationary phases - CSPs) are mostly based on silica gel. In order to separate enantiomers, this is modified with so-called chiral selectors. To date, a large number of such compounds have been developed, resulting in an enormous selection of available phases.
The following classes of compounds are mainly used as chiral selectors:
- So-called "brush phases" - simple chiral molecules, similar to alkyl chains in RP-HPLC, bound to the surface of the silica gel, e.g. dinitrobenzoylphenylglycine
- Helical polymers, e.g. cellulose or amylose derivatives
- Chiral cage compounds, e.g. cyclodextrins or crown ethers
- Proteins, e.g. bovine serum albumin (BSA) or pepsin
- Ligand exchange phases, e.g. amino acid-copper complexes
Modified cellulose or amylose derivatives are most commonly used because these phases can be used to separate a very wide range of enantiomers compared to other chiral selectors.
How are the chiral selectors bound to the silica gel?
There are several possibilities. Some of the chiral selectors can be covalently bound to the silica gel. However, this is not possible for some of the chiral selectors. For example, polysaccharide derivatives are either chemically immobilised to the silica gel ("Immobilized CSPs") or coated onto it ("Coated CSPs") in a special process. The difference between immobilised CSPs and coated CSPs is that the immobilised phases are far more resistant to organic solvents. A number of solvents are not permitted for coated CSPs, as these render the chiral stationary phase unusable over time. This is not the case for immobilised CSPs, which simplifies method development for the separation of enantiomers.
Which mobile phases can be used for chiral separations?
This depends on the stationary phase. As mentioned above, there are chiral phases that are compatible with many organic solvents and others for which the number of possible eluents is very limited. Which eluents may be used can often be found in the column information or "Care&Maintanance" sheets supplied by the manufacturer. If you are unsure, you should ask the respective manufacturer or distributor.
Further tips and information on chiral chromatography
Chiral separations are usually carried out isocratically. As with other chromatographic methods, the resolution for a particular analyte pair is strongly dependent on the selectivity of the stationary phase. Two other important parameters for method development are the mobile phase and the temperature. With chiral separations, it is often the case that the resolution increases with decreasing temperature.
Manufacturers and columns for chiral chromatography
Daicel™ - Chiral Technologies™
Daicel is the leading manufacturer with the largest selection of chiral stationary phases. The available particle sizes range from sub-2 µm for UHPLC separations to 20 µm for MPLC. Chiral TLC plates for thin layer chromatography are also available from Daicel™.
Daicel™ offers the following types of chiral stationary phases (CSPs):
- Polysaccharide based CSPs
- Zwitterionic CSPs
- Protein-based CSPs
- Anion exchange CSPs
- Crown ethers and amino acid-metal complexes
Nouryon™ - Kromasil
With CelluCoat™ and AmyCoat™,Kromasil offers two particularly robust chiral stationary phases. The chiral selector for CelluCoat™ is cellulosetris-(3,5-dimethyl-phenylcarbamate); for AmyCoat™ it is amylosetris-(3,5-dimethyl-phenylcarbamate). The stationary phases withstand pressures up to 400 bar and are compatible with many organic solvents without affecting the performance of the columns. Kromasil® CelluCoat™ and AmyCoat™ are available in particle sizes of 3, 5 or 10 µm, with the 10 µm material also available in bulk.
Macherey-Nagel™
Macherey-Nagel is a manufacturer of several chiral stationary phases for different applications. Chiral TLC plates are also available.
| HPLC column | Chiral selector |
| Nucleodex β-OH | Underivatised β-cyclodextrin |
| Nucleodex β-PM | Permethylated β-cyclodextrin |
| Nucleodex α-PM | Permethylated α-cyclodextrin |
| Nucleocel Delta | Cellulose-tris-(3,5-dimethylphenylcarbamate) |
| Resolvosil BSA-7 | Bovine serum albumin (BSA) |
| Nucleosil CHIRAL-1 | L-Hydroxyproline-Cu2+-complex |
| Nucleosil CHIRAL-2 | N-(3,5-dinitrobenzoyl)-D-phenylglycine |
| Nucleosil CHIRAL-3 | N-(3,5-dinitrobenzoyl)-L-phenylglycine |
Supelco™
Supelco (Merck™ formerly Sigma-Aldrich™) is a manufacturer of several special HPLC columns for enantiomer separation, which were originally developed by the company Astec in collaboration with Prof D. Armstrong from the University of Texas at Arlington. Since 2006, Astec has been part of Sigma-Aldrich, which in turn has been part of Merck KGaA since 2015.
| HPLC column | Chiral selector |
| Astec Chirobiotic® V (100 Å) Astec Chirobiotic® V2 (200 Å) | Vancomycin |
| Astec Chirobiotic® T (100 Å) Astec Chirobiotic® T2 (200 Å) | Teicoplanin |
| Astec Chirobiotic® R (100 Å) | Ristocetin |
| Astec Chirobiotic® TAG (100 Å) | Teicoplanin Aglycone |
| Astec (R,R) P-CAP™ Astec (S,S) P-CAP™ | Poly(trans-1,2-cyclohexanediyl-bis-acrylamide) |
| Astec (R,R) P-CAP™-DP Astec (S,S) P-CAP™-DP | Poly(trans-1,2-diphenyl-bis-acrylamide) |
| Astec CLC-L Astec CLC-D | Amino acid Cu(II)-complexes |
| Astec Cyclobond® I 2000 | β-Cyclodextrin |
| Astec Cyclobond® I 2000 AC | Acetylated β-cyclodextrin |
| HPLC column | Chiral selector |
| Astec Cyclobond® I 2000 DM | 2,3-O-dimethyl β-cyclodextrin |
| Astec Cyclobond® I 2000 DMP | 3,5-dimethylphenyl β-cyclodextrin |
| Astec Cyclobond® I 2000 DMP | 2,6-Dinitro-4-fluoromethylphenyl β-cyclodextrin |
| Astec Cyclobond® I 2000 HP-RSP | R,S-hydroxypropyl β-cyclodextrin |
| Astec Cyclobond® I 2000 RSP | R,S-hydroxypropyl β-cyclodextrin |
| Astec Cyclobond® I 2000 SP | S-hydroxypropyl β-cyclodextrin |
| Astec Cyclobond® II | γ-Cyclodextrin |
| Astec Cyclobond® II AC | Acetylated γ-cyclodextrin |
| Astec Cellulose DMP | Cellulosetris-(3,5-dimethylphenylcarbamate) |
Shinwa Chemical Industries Ltd
Shinwa' s best-selling chromatography column is the Ultron ES-OVM column (Art. No. 401-102), which fulfils the specifications of the United States Pharmacopoeia USP L57. Other columns for chiral chromatography are also available.
| HPLC column | Chiral selector |
| Ultron ES-OVM | Ovomucoid |
| Ultron ES-OVM-C | Ovomucoid |
| Ultron ES-Pepsin | pepsin |
| Ultron ES-BSA | Bovine serum albumin |
| Ultron ES-CD | β-Cyclodextrin |
| Ultron ES-PhCD | β-Cyclodextrin derivative |
Sumika Chemicals Analysis Service Ltd (SCAS)
Sumika™ offers special columns for chiral separations using HPLC.
| Sumichiral | Chiral selector | Sumichiral | Chiral selector |
| OA-2000 | (R)-phenylglycine | OA-2000S | (S)-phenylglycine |
| OA-2000-I | (R)-Phenylglycine | OA-2000S-I | (S)-Phenylglycine |
| OA-2500 | (R)-1-Naphtylglycine | OA-2500S | (S)-1-Naphtylglycine |
| OA-2500-I | (R)-1-Naphtylglycine | OA-2500S-I | (S)-1-Naphthylglycine |
| OA-3100 | (S)-Valine | OA-3100R | (R)-Valine |
| OA-3200 | (S)-tert-Leucine | OA-3200R | (R)-tert-Leucine |
| OA-3300 | (R)-phenylglycine | OA-3300S | (S)-phenylglycine |
| OA-4000 | (S)-Valine (S)-1-(α-naphthyl)ethylamine | OA-4000R | (R)-Valine (R)-1-(α-naphthyl)ethylamine |
| OA-4100 | (S)-Valine (R)-1-(α-naphthyl)ethylamine | OA-4100R | (R)-Valine (S)-1-(α-naphthyl)ethylamine |
| OA-4400 | (S)-Proline (S)-1-(α-naphthyl)ethylamine | OA-4400R | (R)-Proline (R)-1-(α-naphthyl)ethylamine |
| OA-4500 | (S)-Proline (R)-1-(α-naphthyl)ethylamine | OA-4500R | (R)-Proline (S)-1-(α-naphthyl)ethylamine |
| OA-4600 | (S)-tert-Leucine (S)-1-(α-naphthyl)ethylamine | OA-4600R | (R)-tert-Leucine (R)-1-(α-naphthyl)ethylamine |
| OA-4700 | (S)-tert-Leucine (R)-1-(α-naphthyl)ethylamine | OA-4700R | (R)-tert-Leucine (S)-1-(α-naphthyl)ethylamine |
| OA-4800 | (S)-Indoline-2-carboxylic acid (S)-1-(α-naphthyl)ethylamine | - | - |
| OA-4900 | (S)-Indoline-2-carboxylic acid (R)-1-(α-naphthyl)ethylamine | - | - |
| OA-5000 | (D)-Penicillamine | OA-5000L | (L)-Penicillamine |
| OA-6000 | (L)-Tartaric acid (R)-1-(α-Naphthyl)ethylamine | OA-6000R | (D)-Tartaric acid (S)-1-(α-Naphthyl)ethylamine |
| OA-6100 | (L)-Tartaric acid, (S)-Valine (S)-1-(α-Naphthyl)ethylamine | OA-6100R | (D)-Tartaric acid, (R)-Valine (R)-1-(α-Naphthyl)ethylamine |
| OA-7000 | β-Cyclodextrin | - | - |
| OA-7100 | β-Cyclodextrin | - | - |
| OA-7500 | Methyl-β-cyclodexitrin | - | - |
| OA-7600 | Methyl-α-cyclodexitrin | - | - |
| OA-7700 | Acetyl-β-cyclodexitrin | - | - |
| OA-8000 | Chiral pseudo-crown ether | - | - |
Shodex™
| HPLC column | Chiral selector |
| ORpak CDBS-453 | β-Cyclodextrin derivative |
| ORpak CRX-853 | L-amino acid derivative |
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.