- 3% Discount on online orders
- Fast Delivery Times
- DIN ISO 9001:2015 Certified
- Manufacturer Expertise
- Contact Us
Checkout using your account
Checkout as a new customer
Creating an account has many benefits:
- See order and shipping status
- Track order history
- Check out faster
Ligand Exchange Chromatography
Ligand exchange chromatography is a specialised form of chromatography based on the interaction between a metal ion bound to the stationary phase and the analytes that can complex with this metal ion. This technique is mainly used to separate and analyse enantiomers and to separate molecules that are able to form stable complexes with the metal ion, such as certain amino acids, peptides, nucleotides and carbohydrates.
A significant advantage of ligand exchange chromatography is its high enantioselectivity, which makes it particularly valuable for chiral separation, a critical requirement in the pharmaceutical industry. With the ability to effectively separate optical isomers, this method supports drug development and quality control by ensuring that the therapeutically effective enantiomer is separated from potentially harmful isomers. In addition, ligand exchange chromatography offers excellent selectivity and sensitivity for the separation and analysis of biomolecules and metal ion-complexing compounds, making it a valuable tool in biochemical research, environmental analysis and food chemistry.
Products
Technical Data
Basics
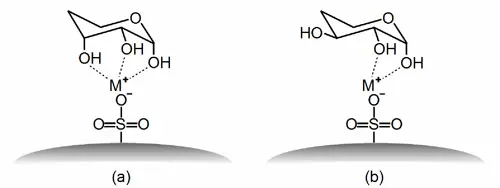
Ligand exchange chromatography is particularly important for analysing sugars. Sulphonate groups are bound to the surface of the stationary phase, which are neutralised to the outside with different metal ions. These metal sulphonates can form complexes with the hydroxyl groups of the sugar molecules. These complexes have different stabilities depending on the type and number of hydroxyl groups involved and the metal ion. The saccharides to be separated are therefore retained to different degrees by the stationary phase and thus eluted at different times.
In Figure 1a, three hydroxyl groups are involved in the formation of a complex. In Figure 1b, only two OH groups can participate in the complex formation due to the spatial arrangement of the hydroxyl groups in the sugar molecule. Thus, the interaction of the sugar molecule with the stationary phase is greater for (a) than for (b).
The ligand exchange mechanism (LEX) is often operated in dual mode with size exclusion chromatography (SEC) or hydrophilic interaction chromatography (HILIC). For the combination of LEX and SEC, only water is used as the mobile phase; for LEX and HILIC, mixtures of acetonitrile and water are used.
The right column for you - we will be happy to support you individually
Competent consultants are always at your side. Write a message to our consultants, we will get back to you and give you individual support.